- Mineral levels closely approximating the mineral content of human milk (60:40 ratio of whey to casein)
- Calcium-to-phosphorus ratio and content designed to manage serum calcium disorders—both hypercalcemia and hypocalcemia due to hyperphosphatemia
- Gluten-free
- Halal
- Kosher
- Gluten-free
- 400-gram can
Similac PM 60/40 Low-Iron Infant Formula Powder
View Questions
(10% off first Autoship order*)
$28.90 – $176.00Price range: $28.90 through $176.00
- FREE 1-2 day shipping on orders $49+
- Free 30-day returns
- 24/7 product guidance
- Mineral levels closely approximating the mineral content of human milk (60:40 ratio of whey to casein)
- Calcium-to-phosphorus ratio and content designed to manage serum calcium disorders—both hypercalcemia and hypocalcemia due to hyperphosphatemia
- Gluten-free
- Halal
- Kosher
- Gluten-free
- 400-gram can
Description
FAQ
Nutrition Facts
About Similac PM 60/40 Low-Iron Infant Formula Powder
Similac PM 60/40 is a specialized infant formula designed to provide balanced nutrition for infants who require reduced mineral intake. With a carefully adjusted calcium-to-phosphorus ratio, this 20 Cal/fl oz formula helps manage serum calcium disorders, including hypercalcemia and hypocalcemia due to hyperphosphatemia, making it suitable for infants with impaired renal function.
Key Benefits & Features
- Specialized Nutrition: Tailored for infants requiring reduced mineral intake.
- Human-Milk-Inspired Mineral Content: Mineral levels are closely aligned with those found in human milk, featuring a 60:40 whey-to-casein ratio.
- Serum Calcium Management: Designed to address serum calcium disorders, including hypercalcemia and hypocalcemia related to hyperphosphatemia.
- Dietary-Friendly: Gluten-free, Halal, and Kosher certified, made with high-quality dairy ingredients.
- Safe Use: For oral or tube feeding only; not suitable for intravenous use.
Similac PM 60/40 provides targeted nutrition to support your infant’s unique dietary needs while ensuring safety and quality.
Similac PM 60/40 Low-Iron Infant Formula Powder FAQs
1. What are the precautions for using this formula?
- Additional iron should be provided from other sources.
- In cases where the infant is losing abnormal amounts of electrolytes, supplementation from other sources may be necessary.
- For low-birth-weight infants (under 1500 g at birth), additional calcium, phosphorus, and sodium may be required during periods of rapid growth.
- Never use a microwave oven to warm the formula—serious burns can occur.
- Not for intravenous (IV) use.
- Not suitable for infants with galactosemia.
2. How do I prepare this formula?
Proper preparation is essential for your baby’s health. Always follow these steps carefully to ensure safety and optimal nutrition:
- Step 1: Wash your hands, surfaces, and all utensils thoroughly.
- Step 2: Pour the desired amount of water into a clean bottle.
- Step 3: Add 1 unpacked, level scoop (8.7 g) of powder for every 2 fl oz of water.
- Step 4: Return the dry scoop to the container.
- Step 5: Cap the bottle, shake well, and attach the nipple.
- Step 6: Begin oral feeding immediately and discard unused formula after 1 hour.
Note: When prepared as directed, one can of formula makes approximately 102 fl oz of formula at 20 Cal/fl oz.
3. How should I store and handle this formula?
- Once mixed, store bottles in the refrigerator and use within 24 hours.
- Store unopened or opened containers at room temperature. Avoid exposing the container to extreme heat or cold.
- Use the contents of an opened container within 1 month.
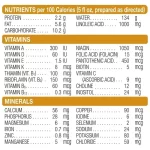
Nutrition Facts
Serving size: 5 fl oz (148 mL)
| Amount per serving | |
|---|---|
| Calories | 100 |
| Protein | 2.2 g |
| Fat | 5.6 g |
| Carbohydrate | 10.2 g |
| Water | 134 g |
| Linoleic Acid | 1000 mg |
| Potential Renal Solute Load | 18.3 mOsm |
| Vitamin A | 300 IU |
| Vitamin D | 60 IU |
| Vitamin E | 1.5 IU |
| Vitamin K | 8 mcg |
| Thiamin (Vitamin B1) | 100 mcg |
| Riboflavin (Vitamin B2) | 150 mcg |
| Vitamin B6 | 60 mcg |
| Vitamin B12 | 0.3 mcg |
| Niacin | 1050 mcg |
| Folic Acid (Folacin) | 15 mcg |
| Pantothenic Acid | 450 mcg |
| Biotin | 5 mcg |
| Vitamin C (Ascorbic Acid) | 9 mg |
| Choline | 12 mg |
| Inositol | 24 mg |
| Calcium | 56 mg |
| Calcium | 2.8 mEq |
| Phosphorus | 28 mg |
| Magnesium | 6 mg |
| Iron | 0.7 mg |
| Zinc | 0.8 mg |
| Manganese | 5 mcg |
| Copper | 90 mcg |
| Iodine | 6 mcg |
| Selenium | 2 mcg |
| Sodium | 24 mg |
| Sodium | 1.0 mEq |
| Potassium | 80 mg |
| Potassium | 2.1 mEq |
| Chloride | 59 mg |
| Chloride | 1.7 mEq |
Additional information
| Select Options |
14.1 oz – 1 Each ,14.1 oz – Case of 6 |
|---|
Reviews (0)
Be the first to review “Similac PM 60/40 Low-Iron Infant Formula Powder” Cancel reply
Resources
Recently Viewed Products
Pedialyte Electrolyte Solution Powder, Multiple Flavors, 0.3 oz., Individual Packet
Rated 0 out of 5
$16.80 – $120.80Price range: $16.80 through $120.80
Enfamil Reguline Infant Formula, Powder, 12.4 oz.
Rated 0 out of 5
$29.40 – $187.60Price range: $29.40 through $187.60
Alfamino Infant Formula With Iron, Amino Acid Based Powder, 14.1 oz
Rated 0 out of 5
$59.50 – $300.80Price range: $59.50 through $300.80
Boudreaux’s Butt Paste Diaper Rash Treatment, Tube, Scented, Multiple Options
Rated 0 out of 5
$7.99 – $11.90Price range: $7.99 through $11.90
Contact Us
Click Now
Monday to Friday 8 am- 6 pm Pacific Time
(800) 924-3560
Join Our Team
Our Story

Join our email list
We’ll send you expert product recommendations, helpful articles, & caregiver stories.
Monday to Friday 8 am- 6 pm Pacific Time





































































































Reviews
There are no reviews yet.